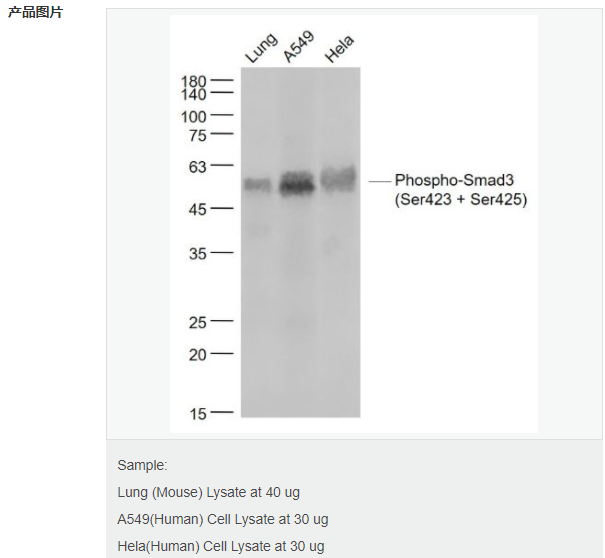
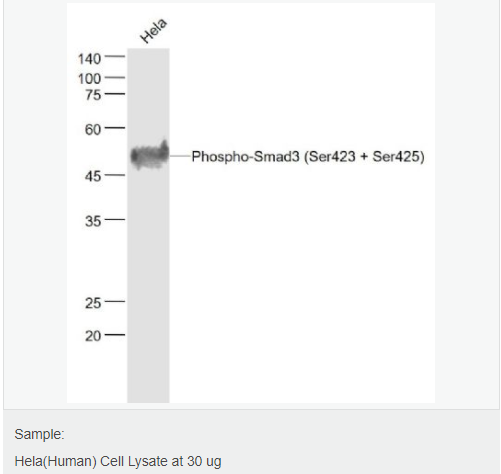
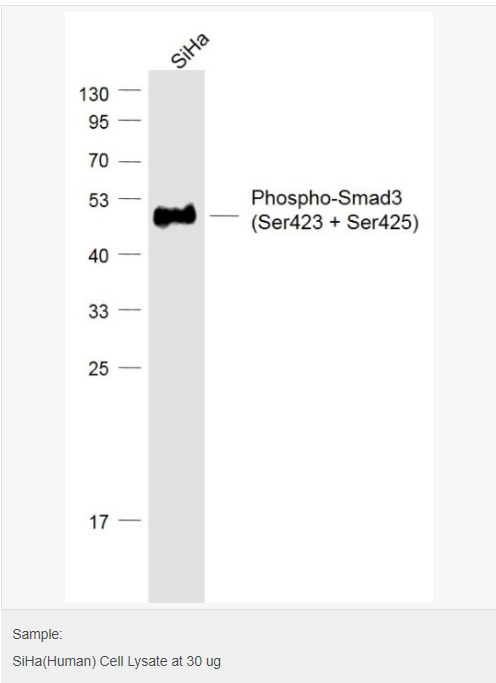
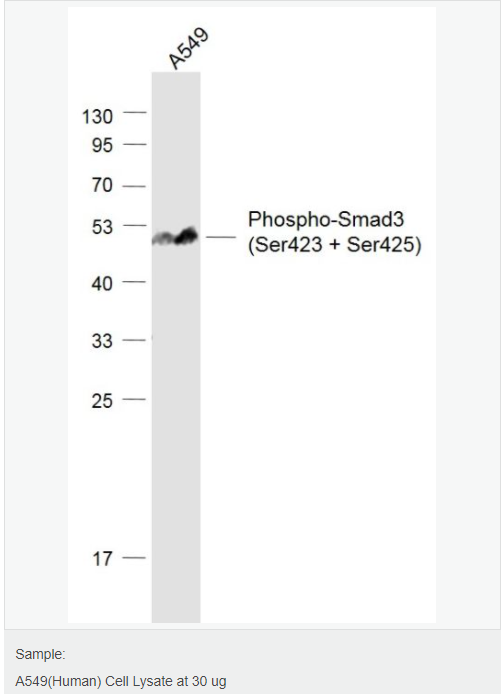
货号
产品规格
售价
备注
BN42104R-50ul
50ul
¥2020.00
交叉反应:Human,Mouse(predicted:Rat,Pig) 推荐应用:WB,IHC-P,IHC-F,IF
BN42104R-100ul
100ul
¥3240.00
交叉反应:Human,Mouse(predicted:Rat,Pig) 推荐应用:WB,IHC-P,IHC-F,IF
| 英文名称 | Phospho-Smad3 (Ser423 + Ser425) |
| 中文名称 | 磷酸化细胞信号转导分子SMAD3重组兔单克隆抗体 |
| 别 名 | Smad3 (phospho Ser423/425); Smad3 (phospho S423 + S425); p-Smad3 (Ser423 + Ser425); hMAD 3; hSMAD3; HSPC193; JV15 2; JV152; MAD (mothers against decapentaplegic Drosophila) homolog 3; MAD3; MADH 3; MADH3; Mothers against decapentaplegic homolog 3; Mothers against DPP homolog 3; SMA and MAD related protein 3; SMAD 3; SMAD; SMAD-3; SMAD3_HUMAN. |
| 产品类型 | 磷酸化抗体 |
| 研究领域 | 肿瘤 细胞生物 免疫学 信号转导 干细胞 细胞凋亡 转录调节因子 表观遗传学 |
| 抗体来源 | Rabbit |
| 克隆类型 | Monoclonal |
| 克 隆 号 | 5C5 |
| 交叉反应 | Human, Mouse, (predicted: Rat, Pig, ) |
| 产品应用 | WB=1:500-2000 IHC-P=1:20-200 IHC-F=1:20-200 ICC=1:20-200 IF=1:20-200 (石蜡切片需做抗原修复) not yet tested in other applications. optimal dilutions/concentrations should be determined by the end user. |
| 分 子 量 | 47kDa |
| 细胞定位 | 细胞核 细胞浆 |
| 性 状 | Liquid |
| 浓 度 | 1mg/ml |
| 免 疫 原 | KLH conjugated Synthesised phosphopeptide derived from human Smad3 around the phosphorylation site of Ser423/425:CS(p-S)V(p-S) |
| 亚 型 | IgG |
| 纯化方法 | affinity purified by Protein A |
| 储 存 液 | 0.01M TBS(pH7.4) with 1% BSA, 0.03% Proclin300 and 50% Glycerol. |
| 保存条件 | Shipped at 4℃. Store at -20 °C for one year. Avoid repeated freeze/thaw cycles. |
| PubMed | PubMed |
| 产品介绍 | Smad3 is a 50 kDa member of a family of proteins that act as key mediators of TGF beta superfamily signaling in cell proliferation, differentiation and development. The Smad family is divided into three subclasses: receptor regulated Smads, activin/TGF beta receptor regulated (Smad2 and 3) or BMP receptor regulated (Smad 1, 5, and 8); the common partner, (Smad4) that functions via its interaction to the various Smads; and the inhibitory Smads, (Smad6 and 7). Activated Smad3 oligomerizes with Smad4 upon TGF beta stimulation and translocates as a complex into the nucleus, allowing its binding to DNA and transcription factors. Phosphorylation of the two TGF beta dependent serines 423 and 425 in the C terminus of Smad3 is critical for Smad3 transcriptional activity and TGF beta signaling. Function: Receptor-regulated SMAD (R-SMAD) that is an intracellular signal transducer and transcriptional modulator activated by TGF-beta (transforming growth factor) and activin type 1 receptor kinases. Binds the TRE element in the promoter region of many genes that are regulated by TGF-beta and, on formation of the SMAD3/SMAD4 complex, activates transcription. Also can form a SMAD3/SMAD4/JUN/FOS complex at the AP-1/SMAD site to regulate TGF-beta-mediated transcription. Has an inhibitory effect on wound healing probably by modulating both growth and migration of primary keratinocytes and by altering the TGF-mediated chemotaxis of monocytes. This effect on wound healing appears to be hormone-sensitive. Regulator of chondrogenesis and osteogenesis and inhibits early healing of bone fractures (By similarity). Positively regulates PDPK1 kinase activity by stimulating its dissociation from the 14-3-3 protein YWHAQ which acts as a negative regulator. Subunit: Monomer; in the absence of TGF-beta. Homooligomer; in the presence of TGF-beta. Heterotrimer; forms a heterotrimer in the presence of TGF-beta consisting of two molecules of C-terminally phosphorylated SMAD2 or SMAD3 and one of SMAD4 to form the transcriptionally active SMAD2/SMAD3-SMAD4 complex. Interacts with TGFBR1. Part of a complex consisting of AIP1, ACVR2A, ACVR1B and SMAD3. Interacts with AIP1, TGFB1I1, TTRAP, FOXL2, PML, PRDM16, HGS and WWP1. Interacts (via MH2 domain) with CITED2 (via C-terminus) (By similarity). Interacts with NEDD4L; the interaction requires TGF-beta stimulation (By similarity). Interacts (via the MH2 domain) with ZFYVE9. Interacts with HDAC1, VDR, TGIF and TGIF2, RUNX3, CREBBP, SKOR1, SKOR2, SNON, ATF2, SMURF2 and TGFB1I1. Interacts with DACH1; the interaction inhibits the TGF-beta signaling. Forms a complex with SMAD2 and TRIM33 upon addition of TGF-beta. Found in a complex with SMAD3, RAN and XPO4. Interacts in the complex directly with XPO4. Interacts (via the MH2 domain) with LEMD3; the interaction represses SMAD3 transcriptional activity through preventing the formation of the heteromeric complex with SMAD4 and translocation to the nucleus. Interacts with RBPMS. Interacts (via MH2 domain) with MECOM. Interacts with WWTR1 (via its coiled-coil domain). Interacts (via the linker region) with EP300 (C-terminal); the interaction promotes SMAD3 acetylation and is enhanced by TGF-beta phosphorylation in the C-terminal of SMAD3. This interaction can be blocked by competitive binding of adenovirus oncoprotein E1A to the same C-terminal site on EP300, which then results in partially inhibited SMAD3/SMAD4 transcriptional activity. Interacts with SKI; the interaction represses SMAD3 transcriptional activity. Component of the multimeric complex SMAD3/SMAD4/JUN/FOS which forms at the AP1 promoter site; required for syngernistic transcriptional activity in response to TGF-beta. Interacts (via an N-terminal domain) with JUN (via its basic DNA binding and leucine zipper domains); this interaction is essential for DNA binding and cooperative transcriptional activity in response to TGF-beta. Interacts with PPM1A; the interaction dephosphorylates SMAD3 in the C-terminal SXS motif leading to disruption of the SMAD2/3-SMAD4 complex, nuclear export and termination of TGF-beta signaling. Interacts (dephosphorylated form via the MH1 and MH2 domains) with RANBP3 (via its C-terminal R domain); the interaction results in the export of dephosphorylated SMAD3 out of the nucleus and termination of the TGF-beta signaling. Interacts with MEN1. Interacts with IL1F7. Interaction with CSNK1G2. Interacts with PDPK1 (via PH domain). Subcellular Location: Cytoplasm. Nucleus. Cytoplasmic and nuclear in the absence of TGF-beta. On TGF-beta stimulation, migrates to the nucleus when complexed with SMAD4. Through the action of the phosphatase PPM1A, released from the SMAD2/SMAD4 complex, and exported out of the nucleus by interaction with RANBP1. Co-localizes with LEMD3 at the nucleus inner membrane. MAPK-mediated phosphorylation appears to have no effect on nuclear import. PDPK1 prevents its nuclear translocation in response to TGF-beta. Post-translational modifications: Phosphorylated on serine and threonine residues. Enhanced phosphorylation in the linker region on Thr-179, Ser-204 and Ser-208 on EGF AND TGF-beta treatment. Ser-208 is the main site of MAPK-mediated phosphorylation. CDK-mediated phosphorylation occurs in a cell-cycle dependent manner and inhibits both the transcriptional activity and antiproliferative functions of SMAD3. This phosphorylation is inhibited by flavopiridol. Maximum phosphorylation at the G(1)/S junction. Also phosphorylated on serine residues in the C-terminal SXS motif by TGFBR1 and ACVR1. TGFBR1-mediated phosphorylation at these C-terminal sites is required for interaction with SMAD4, nuclear location and transactivational activity, and appears to be a prerequisite for the TGF-beta mediated phosphorylation in the linker region. Dephosphorylated in the C-terminal SXS motif by PPM1A. This dephosphorylation disrupts the interaction with SMAD4, promotes nuclear export and terminates TGF-beta-mediated signaling. Phosphorylation at Ser-418 by CSNK1G2/CK1 promotes ligand-dependent ubiquitination and subsequent proteasome degradation, thus inhibiting SMAD3-mediated TGF-beta responses. Phosphorylated by PDPK1. Acetylation in the nucleus by EP300 in the MH2 domain regulates positively its transcriptional activity and is enhanced by TGF-beta. Ubiquitinated. DISEASE: Defects in SMAD3 may be a cause of colorectal cancer (CRC) [MIM:114500]. Defects in SMAD3 are the cause of Loeys-Dietz syndrome type 1C (LDS1C) [MIM:613795]. An aortic aneurysm syndrome with widespread systemic involvement. The disorder is characterized by the triad of arterial tortuosity and aneurysms, hypertelorism, and bifid uvula or cleft palate. Patients with LDS1C also manifest early-onset osteoarthritis. They lack craniosynostosis and mental retardation. Note=SMAD3 mutations have been reported to be also associated with thoracic aortic aneurysms and dissection (TAAD) (PubMed:21778426). This phenotype is distinguised from LDS1C by having aneurysms restricted to thoracic aorta. As individuals carrying these mutations also exhibit aneurysms of other arteries, including abdominal aorta, iliac, and/or intracranial arteries (PubMed:21778426), they have been classified as LDS1C by the OMIM resource. Similarity: Belongs to the dwarfin/SMAD family. Contains 1 MH1 (MAD homology 1) domain. Contains 1 MH2 (MAD homology 2) domain. SWISS: P84022 Gene ID: 4088 Database links: Entrez Gene: 4088 Human Entrez Gene: 17127 Mouse Omim: 603109 Human SwissProt: P84022 Human SwissProt: Q8BUN5 Mouse Unigene: 727986 Human Unigene: 7320 Mouse Unigene: 10636 Rat Important Note: This product as supplied is intended for research use only, not for use in human, therapeutic or diagnostic applications. |