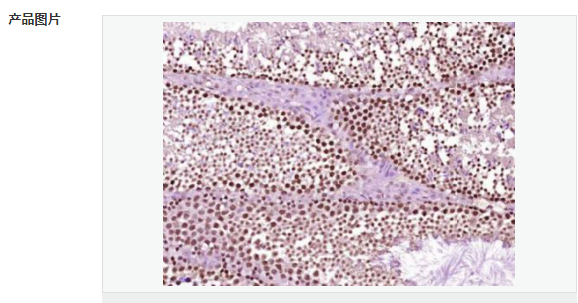
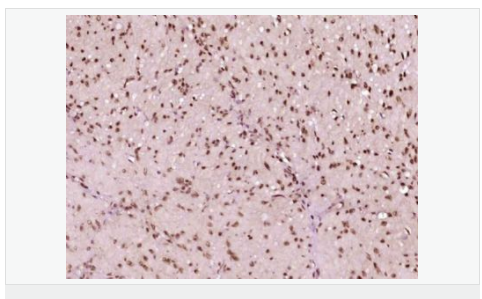
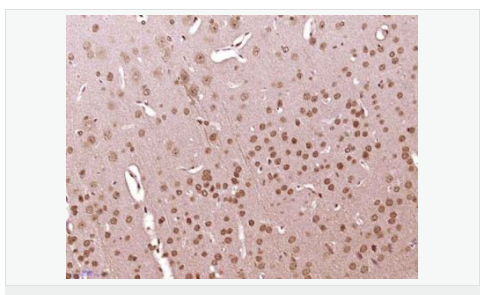
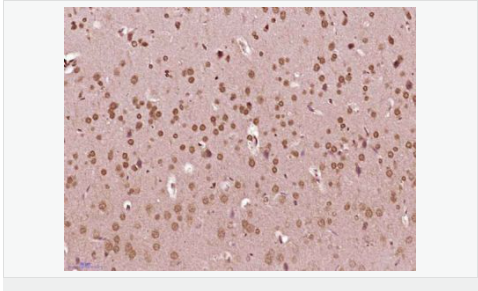
货号
产品规格
售价
备注
BN40576R-100ul
100ul
¥2470.00
交叉反应:Human,Mouse,Rat(predicted:Chicken,Dog,Pig,Cow,Rabbit,Sheep) 推荐应用:IHC-P,IHC-F,IF,ELISA
| 英文名称 | phospho-Src (Ser75) |
| 中文名称 | 磷酸化src原癌基因抗体 |
| 别 名 | Src (phospho S75); Src (phospho Ser75); p-Src (phospho S75); ASV; Avian sarcoma virus; c SRC; CDNA FLJ14219 fis clone NT2RP3003800 highly similar to Rattus norvegicus tyrosine protein kinase pp60 c src mRNA; cSrc; EC 2.7.10.2; Neuronal CSRC tyrosine specific protein kinase; Neuronal SRC; Oncogene SRC; OTTHUMP00000174476; OTTHUMP00000174477; p60 Src; p60-Src; p60Src; pp60c src; pp60c-src; pp60csrc; Proto oncogene tyrosine protein kinase Src; Proto-oncogene c-Src; Proto-oncogene tyrosine-protein kinase Src; Protooncogene SRC; Protooncogene SRC Rous sarcoma; Src; SRC Oncogene; SRC proto oncogene non receptor tyrosine kinase; SRC_HUMAN; SRC1; Tyrosine kinase pp60c src; Tyrosine protein kinase SRC 1; Tyrosine protein kinase SRC1; v src avian sarcoma (Schmidt Ruppin A2) viral oncogene homolog; V src sarcoma (Schmidt Ruppin A 2) viral oncogene homolog (avian); v src sarcoma (Schmidt Ruppin A 2) viral oncogene homolog avian. |
| 产品类型 | 磷酸化抗体 |
| 研究领域 | 肿瘤 细胞生物 信号转导 转录调节因子 激酶和磷酸酶 表观遗传学 |
| 抗体来源 | Rabbit |
| 克隆类型 | Polyclonal |
| 交叉反应 | Human, Mouse, Rat, (predicted: Chicken, Dog, Pig, Cow, Rabbit, Sheep, ) |
| 产品应用 | ELISA=1:5000-10000 IHC-P=1:100-500 IHC-F=1:100-500 ICC=1:100-500 IF=1:200-800 (石蜡切片需做抗原修复) not yet tested in other applications. optimal dilutions/concentrations should be determined by the end user. |
| 分 子 量 | 61kDa |
| 细胞定位 | 细胞膜 |
| 性 状 | Liquid |
| 浓 度 | 1mg/ml |
| 免 疫 原 | KLH conjugated synthesised phosphopeptide derived from human Src around the phosphorylation site of Ser75:VT(p-S)PQ |
| 亚 型 | IgG |
| 纯化方法 | affinity purified by Protein A |
| 储 存 液 | 0.01M TBS(pH7.4) with 1% BSA, 0.03% Proclin300 and 50% Glycerol. |
| 保存条件 | Shipped at 4℃. Store at -20 °C for one year. Avoid repeated freeze/thaw cycles. |
| PubMed | PubMed |
| 产品介绍 | This gene is highly similar to the v-src gene of Rous sarcoma virus. This proto-oncogene may play a role in the regulation of embryonic development and cell growth. The protein encoded by this gene is a tyrosine-protein kinase whose activity can be inhibited by phosphorylation by c-SRC kinase. Mutations in this gene could be involved in the malignant progression of colon cancer. Two transcript variants encoding the same protein have been found for this gene. [provided by RefSeq, Jul 2008] Function: Non-receptor protein tyrosine kinase which is activated following engagement of many different classes of cellular receptors including immune response receptors, integrins and other adhesion receptors, receptor protein tyrosine kinases, G protein-coupled receptors as well as cytokine receptors. Participates in signaling pathways that control a diverse spectrum of biological activities including gene transcription, immune response, cell adhesion, cell cycle progression, apoptosis, migration, and transformation. Due to functional redundancy between members of the SRC kinase family, identification of the specific role of each SRC kinase is very difficult. SRC appears to be one of the primary kinases activated following engagement of receptors and plays a role in the activation of other protein tyrosine kinase (PTK) families. Receptor clustering or dimerization leads to recruitment of SRC to the receptor complexes where it phosphorylates the tyrosine residues within the receptor cytoplasmic domains. Plays an important role in the regulation of cytoskeletal organization through phosphorylation of specific substrates such as AFAP1. Phosphorylation of AFAP1 allows the SRC SH2 domain to bind AFAP1 and to localize to actin filaments. Cytoskeletal reorganization is also controlled through the phosphorylation of cortactin (CTTN). When cells adhere via focal adhesions to the extracellular matrix, signals are transmitted by integrins into the cell resulting in tyrosine phosphorylation of a number of focal adhesion proteins, including PTK2/FAK1 and paxillin (PXN). In addition to phosphorylating focal adhesion proteins, SRC is also active at the sites of cell-cell contact adherens junctions and phosphorylates substrates such as beta-catenin (CTNNB1), delta-catenin (CTNND1), and plakoglobin (JUP). Another type of cell-cell junction, the gap junction, is also a target for SRC, which phosphorylates connexin-43 (GJA1). SRC is implicated in regulation of pre-mRNA-processing and phosphorylates RNA-binding proteins such as KHDRBS1. Also plays a role in PDGF-mediated tyrosine phosphorylation of both STAT1 and STAT3, leading to increased DNA binding activity of these transcription factors. Involved in the RAS pathway through phosphorylation of RASA1 and RASGRF1. Plays a role in EGF-mediated calcium-activated chloride channel activation. Required for epidermal growth factor receptor (EGFR) internalization through phosphorylation of clathrin heavy chain (CLTC and CLTCL1) at 'Tyr-1477'. Involved in beta-arrestin (ARRB1 and ARRB2) desensitization through phosphorylation and activation of ADRBK1, leading to beta-arrestin phosphorylation and internalization. Has a critical role in the stimulation of the CDK20/MAPK3 mitogen-activated protein kinase cascade by epidermal growth factor. Might be involved not only in mediating the transduction of mitogenic signals at the level of the plasma membrane but also in controlling progression through the cell cycle via interaction with regulatory proteins in the nucleus. Plays an important role in osteoclastic bone resorption in conjunction with PTK2B/PYK2. Both the formation of a SRC-PTK2B/PYK2 complex and SRC kinase activity are necessary for this function. Recruited to activated integrins by PTK2B/PYK2, thereby phosphorylating CBL, which in turn induces the activation and recruitment of phosphatidylinositol 3-kinase to the cell membrane in a signaling pathway that is critical for osteoclast function. Promotes energy production in osteoclasts by activating mitochondrial cytochrome C oxidase. Phosphorylates DDR2 on tyrosine residues, thereby promoting its subsequent autophosphorylation. Phosphorylates RUNX3 and COX2 on tyrosine residues, TNK2 on 'Tyr-284' and CBL on 'Tyr-731'. Enhances DDX58/RIG-I-elicited antiviral signaling. Phosphorylates PDPK1 at 'Tyr-9', 'Tyr-373' and 'Tyr-376'. Phosphorylates BCAR1 at 'Tyr-132'. Subunit: Interacts with CDCP1, PELP1, TGFB1I1 and TOM1L2. Interacts with DDEF1/ASAP1 via its SH3 domain. Interacts with CCPG1. Interacts with the cytoplasmic domain of MUC1, phosphorylates it and increases binding of MUC1 with beta-catenin. Interacts with RALGPS1 via its SH3 domain. Interacts with CAV2 (tyrosine phosphorylated form). Interacts (via the SH3 domain and the protein kinase domain) with ARRB1; the interaction is independent of the phosphorylation state of SRC C-terminus. Interacts with FCAMR and PXN. Interacts with ARRB2. Interacts with ARRB1. Interacts with SRCIN1. Interacts with SRCIN1. Interacts with NDFIP2 and more weakly with NDFIP1. Interacts with PIK3CA and/or PIK3C2B, PTK2/FAK1, ESR1 (dimethylated on arginine) and FAK. Interacts (via SH2 and SH3 domain) with TNK2. Interacts (via protein kinase domain) with the tyrosine phosphorylated form of RUNX3 (via runt domain). Interacts with TRAF3 (via RING-type zinc finger domain). Interacts with DDX58, MAVS and TBK1. Interacts (via SH2 domain) with GNB2L1/RACK1; the interaction is enhanced by tyrosine phosphorylation of GNB2L1 and inhibits SRC activity. Interacts (via SH2 domain) with the 'Tyr-402' phosphorylated form of PTK2B/PYK2. Interacts (via SH2 domain) with FLT3 (tyrosine phosphorylated). Identified in a complex containing FGFR4, NCAM1, CDH2, PLCG1, FRS2, SRC, SHC1, GAP43 and CTTN. Interacts with EPHB1; activates the MAPK/ERK cascade to regulate cell migration. Interacts with ERBB2 and STAT1. Interacts with PDGFRA (tyrosine phosphorylated). Interacts with CSF1R. Interacts (via SH2 domain) with the 'Tyr-9' phosphorylated form of PDPK1. Interacts with DDR2. Interacts with AMOTL2; this interaction regulates the translocation of phosphorylated SRC to peripheral cell-matrix adhesion sites. Interacts with DDR1 and DAB2. Subcellular Location: Cell membrane. Mitochondrion inner membrane. Nucleus. Cytoplasm, cytoskeleton. Note=Localizes to focal adhesion sites after integrin engagement. Localization to focal adhesion sites requires myristoylation and the SH3 domain. Tissue Specificity: Expressed ubiquitously. Platelets, neurons and osteoclasts express 5-fold to 200-fold higher levels than most other tissues. Post-translational modifications: Myristoylated at Gly-2, and this is essential for targeting to membranes. Dephosphorylated at Tyr-535 by PTPRJ. Phosphorylated on Tyr-535 by c-Src kinase (CSK). The phosphorylated form is termed pp60c-src. Dephosphorylated by PTPRJ at Tyr-424. Normally maintained in an inactive conformation with the SH2 domain engaged with Tyr-535, the SH3 domain engaged with the SH2-kinase linker, and Tyr-424 dephosphorylated. Dephosphorylation of Tyr-535 as a result of protein tyrosine phosphatase (PTP) action disrupts the intramolecular interaction between the SH2 domain and Tyr-535, Tyr-424 can then become autophosphorylated, resulting in SRC activation. Phosphorylation of Tyr-535 by CSK allows this interaction to reform, resulting in SRC inactivation. CDK5-mediated phosphorylation at Ser-74 targets SRC to ubiquitin-dependent degradation and thus leads to cytoskeletal reorganization. Phosphorylated by PTK2/FAK1; this enhances kinase activity. Phosphorylated by PTK2B/PYK2; this enhances kinase activity. S-nitrosylation is important for activation of its kinase activity. Ubiquitinated in response to CDK5-mediated phosphorylation. Similarity: Belongs to the protein kinase superfamily. Tyr protein kinase family. SRC subfamily. Contains 1 protein kinase domain. Contains 1 SH2 domain. Contains 1 SH3 domain. SWISS: P12931 Gene ID: 6714 Database links: Entrez Gene: 6714 Huma Entrez Gene: 20779 Mouse Omim: 190090 Human SwissProt: P12931 Human SwissProt: P05480 Mouse Unigene: 195659 Human Unigene: 22845 Mouse Unigene: 112600 Rat Important Note: This product as supplied is intended for research use only, not for use in human, therapeutic or diagnostic applications. |